The Ministry of Health held DHF Day 2023 yesterday, a training aimed at all provincial health teams.
The activity featured a lecture from Dr. Virginia Bonardo, a well-known researcher working on the process of applying a vaccine to prevent dengue fever, which will be implemented in the country.
The talk was held in the Auditorium Hall of the Corrientes Institute of Cardiology. The modality is hybrid. In person, the Region 1 health team was present and, virtually, the entire province.
“Today we conducted a training aimed at all provincial health teams to evaluate our dengue outbreak in Corrientes, ending with the current outbreak which is still ongoing. In addition, clinical cases of all patients requiring hospitalization are also presented”, said the general director of Epidemiology, Angelina Bobadilla.
“In this case, an evaluation was made of how the virus behaves clinically and, finally, we have a presentation from one of the researchers working on a Dengue vaccine approved by the National Medicines, Food and Technology Administration Médica ( Anmat)”, he added and indicated that such a vaccine should arrive at Corrientes towards the end of the year.
For her part, the director of Vector Diseases and Zoonoses, Lilian Percíncula, points out: “This health system linkage, epidemiology, is very important for action plans such as primary health care.”
Finally, Julio Vallejos, director of the Institute of Cardiology of Corrientes, stated: “It is an honor that this conference was held at our institution, to discuss the knowledge that will allow us to develop strategies to treat pathologies such as dengue.” .
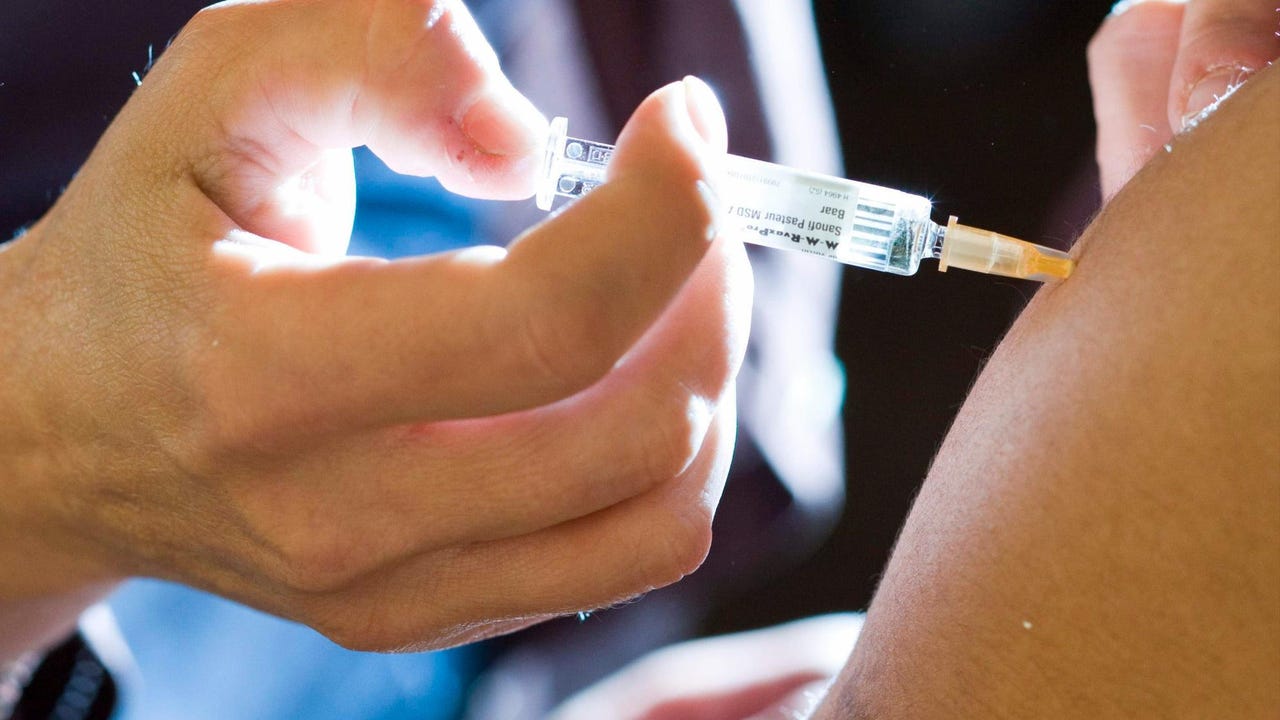
Vaccine
Called TAK-003, the vaccine is based on the dengue virus 2, to which DNA from three other serotypes is added to protect against one of the four types of dengue fever. The form of administration consists of two doses that must be applied three months apart.
TAK-003, also known as Qdenga, received its first approval in August 2022 in Indonesia and then in the European Union in December 2022. It was followed by the UK in January 2023 and was also recently approved by Brazil’s National Health Surveillance Agency (Anvisa).
Its use is intended for endemic areas. After being approved by Annat, the Japanese laboratory will establish a protocol to start the production process for batch shipment to our country.
When this happens, once this cycle is complete and always adhering to quality and traceability standards, Argentina will be in a position to accept the consignment for further commercialization in that country.

“Internet trailblazer. Troublemaker. Passionate alcohol lover. Beer advocate. Zombie ninja.”






/cloudfront-us-east-1.images.arcpublishing.com/eluniverso/FTEC73B3HFHWHOUJFHJPCYHHY4.jpg)