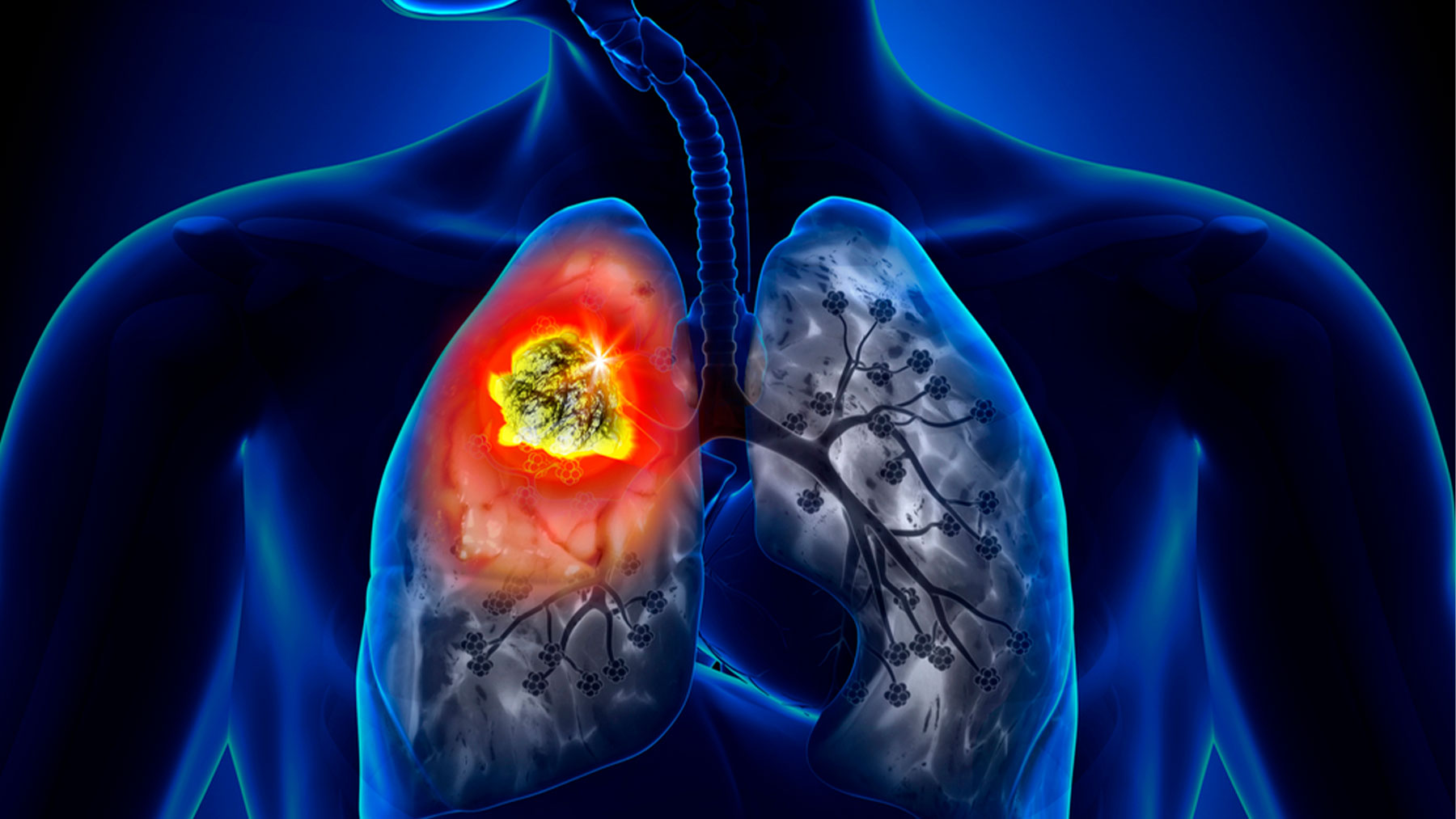
The European Commission has approved atezolizumab, registered by Roche under the name “Tecentriq”, as an adjunct treatment, after complete surgery and platinum-based chemotherapy, for adults with non-small cell lung cancer (NSCLC) at high risk of recurrence whose tumors exhibit 50 percent PD-L1 and who do not have NSCLC EGFR or ALK-positive mutations.
“Today’s approval is an important step forward as ‘Tecentriq’ is the first cancer immunotherapy approved in Europe for the treatment of certain types of early-stage NSCLC. Given that about half of people with early-stage NSCLC experience a relapse after surgery, which in some cases is no longer curable, treating these tumors at an early stage offers the best chance of preventing these recurrences,” he said. Chief Medical Officer and Head of Global Product Development at Roche, Levi Garraway.
The approval is based on the results of an interim analysis of phase III study «IMpower010». The results showed that treatment with “Tecentriq” after complete surgery and platinum-based chemotherapy reduced the risk of disease recurrence or death by 57 percent in people with stage II-IIIA NSCLC whose tumors showed PD. ALK-positive, compared with best supportive care (BSC). Inform EP.
A disease-free survival benefit was seen across most of the subgroups, including histology or disease stage, with the Tecentriq adjuvant compared with BSC. Overall survival (OS) data for patients with resectable stage II-IIIA PD-L1 disease, and without EGFR or ALK-positive mutations, are immature and have not been formally tested in interim analysis of disease-free survival. an OS improvement trend was observed with “Tecentriq”, with a stratified HR of 0.39.
The follow-up will continue with a planned analysis of the more mature SG data later this year. The security data for ‘Tecentriq’ is consistent with known security profiles and no new security signals have been identified.
Approved in 19 countries
To date, “Tecentriq” has been approved in 19 countries, including the United States and China, as an adjunct treatment, after complete intervention and chemotherapy, for adults with stage II-IIIA NSCLC whose tumors express one percent PD-L1. In addition, it has also been approved in three other countries, including Canada and the United Kingdom, as an adjunct treatment after full intervention and chemotherapy, for adult patients with stage II-IIIA NSCLC whose tumors have PD-L1 expression in 50 percent of cases. .
“Tecentriq” has shown significant clinical benefit in various types of lung cancer, with six indications currently approved in countries around the world. It was the first cancer immunotherapy approved for first-line treatment of adults with advanced small cell lung cancer (SCLC), in combination with carboplatin and etoposide (chemotherapy).
It also has four approved indications for advanced or metastatic NSCLC, as a single agent or in combination with targeted therapy and/or chemotherapy. It is available in three dosage options, providing flexibility to choose to administer every two, three or four weeks.

“Internet trailblazer. Troublemaker. Passionate alcohol lover. Beer advocate. Zombie ninja.”


/cloudfront-us-east-1.images.arcpublishing.com/eluniverso/FTEC73B3HFHWHOUJFHJPCYHHY4.jpg)
:quality(85)/cloudfront-us-east-1.images.arcpublishing.com/infobae/3FBT6VVNRDO6XEXJQM5YMGSWSU.jpg)

